IQVIA supports sites and participants through patient-centric trial experiences
Por um escritor misterioso
Descrição
In this interview, Eric Klaver, the DCT regulatory director for decentralized clinical trials at IQVIA, aiding in compliance of the DCT strategy and platform, shares how IQVIA supports investigator | With IQVIA’s clinical trial solutions and flexible site support strategies, decentralized trial elementss are being leveraged to enhance patient- centered care as well as support sites through hands- on enablement and implementation of DCT platforms these services.
Patient Engagement Suite - IQVIA

CRO Trends: IQVIA Perspective

Flexible Clinical Modeling: How Advanced Analytics and AI/ML Can Help Ensure Effective Patient-Centered Drug Development

Patient reported outcome assessment must be inclusive and equitable

Patient Engagement Suite - IQVIA

Oncology Clinical Trials Oncology Clinical Research - IQVIA Biotech

Enrollment Surpasses Projections In International Psoriasis Studies
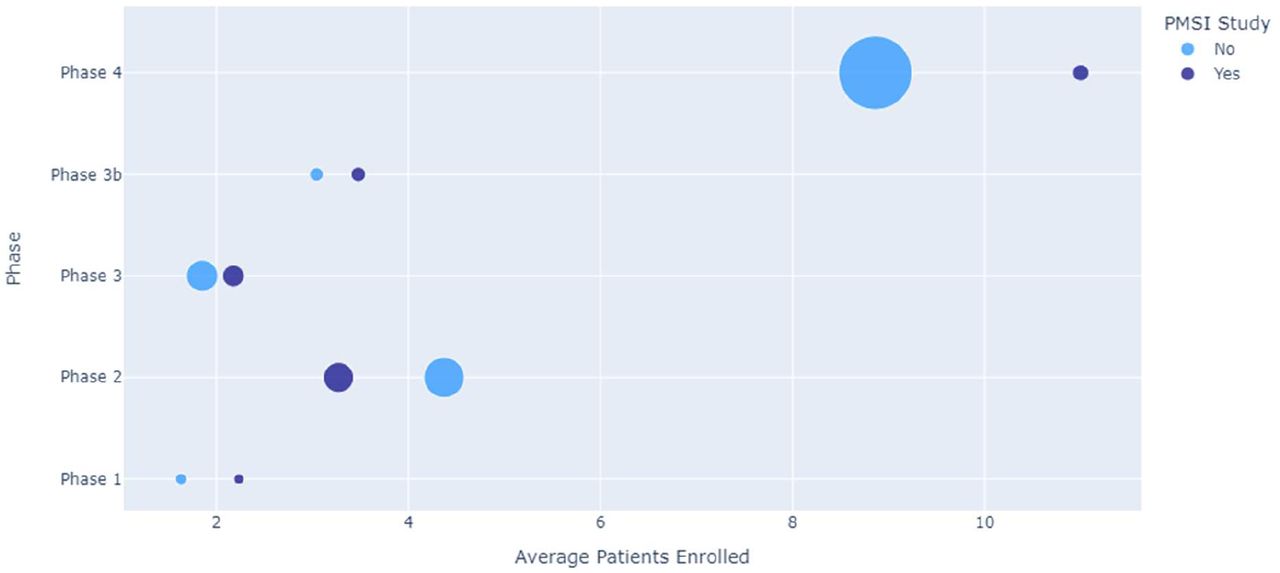
An observational analysis of patient recruitment in clinical trials in France using real-word database PMSI

Iqvia launches patient portal that provides trial participants with study updates, outcomes

WEBINAR: Patient centric sampling in pediatric clinical trials – successes, challenges and how to overcome them - The Evidence Base
de
por adulto (o preço varia de acordo com o tamanho do grupo)






